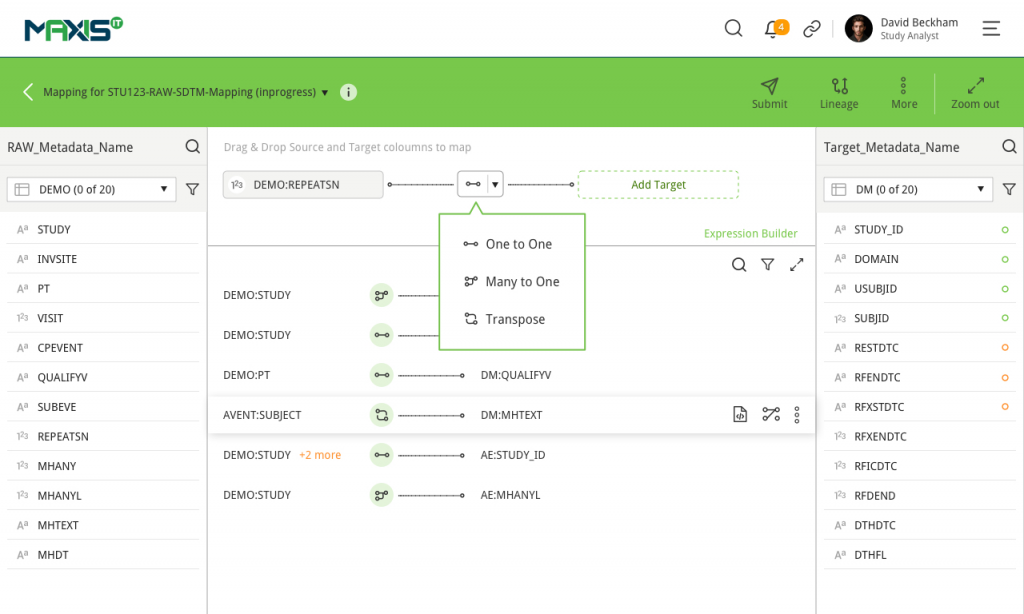
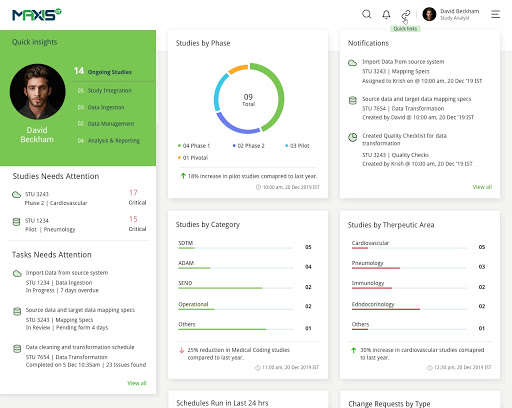
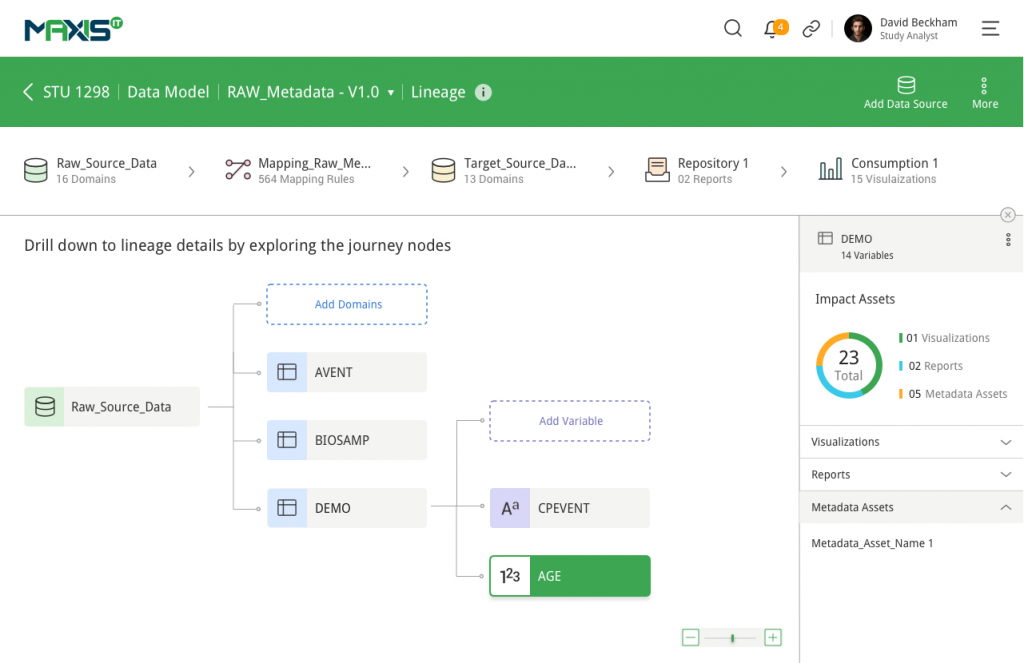
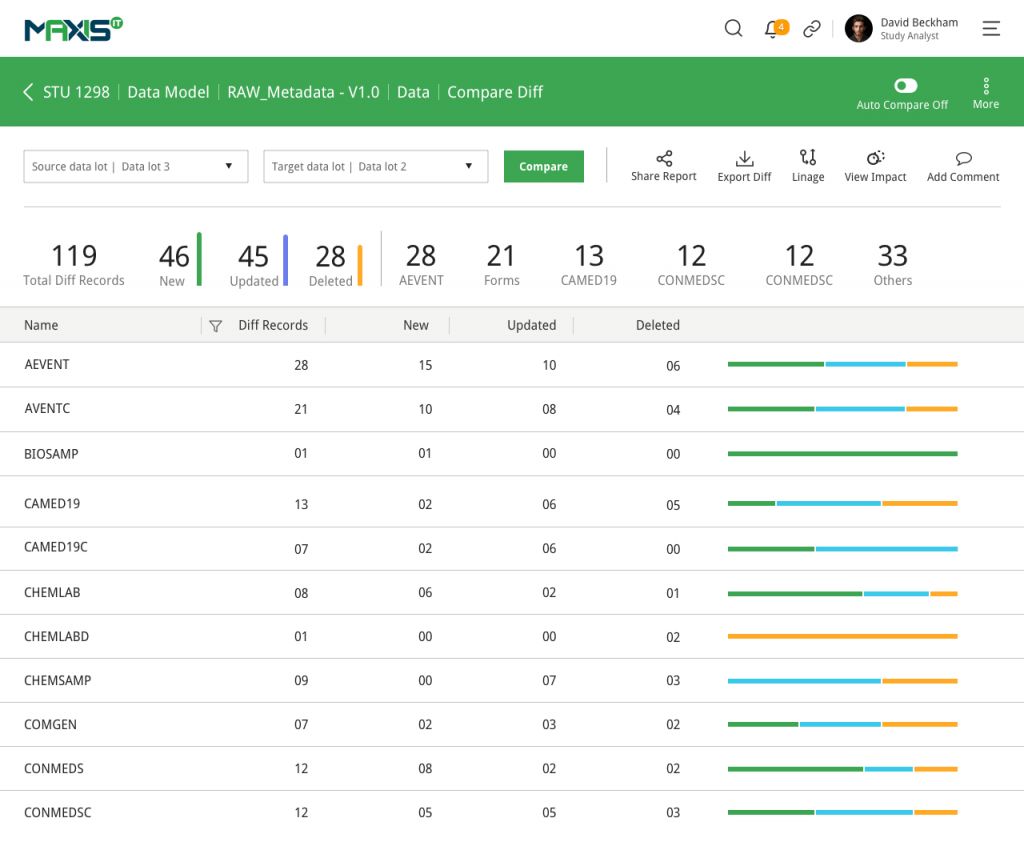
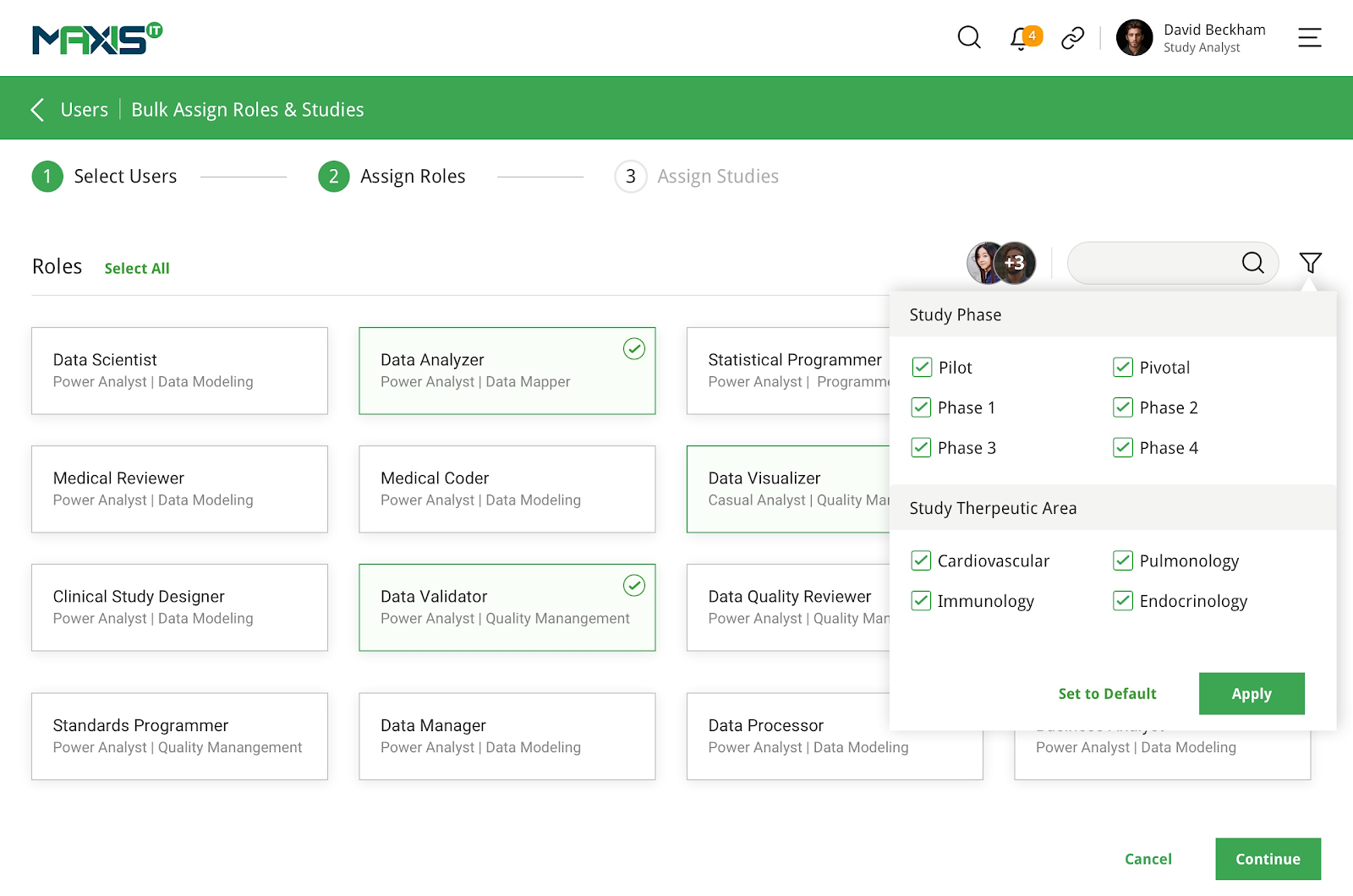
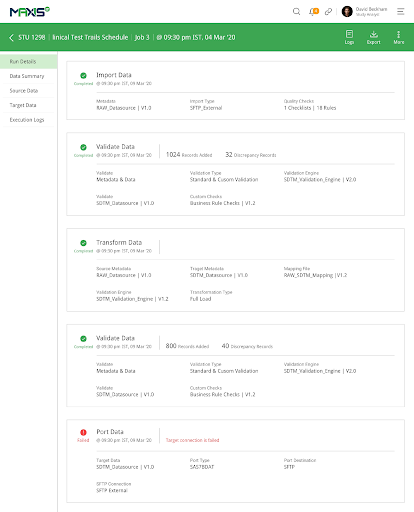
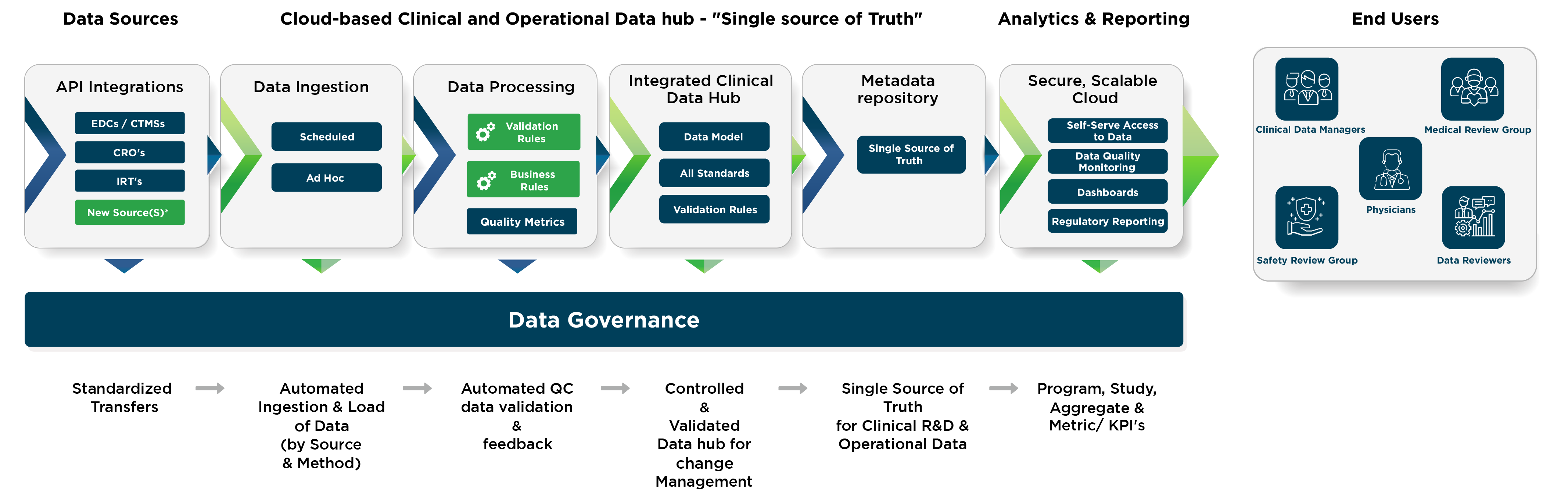
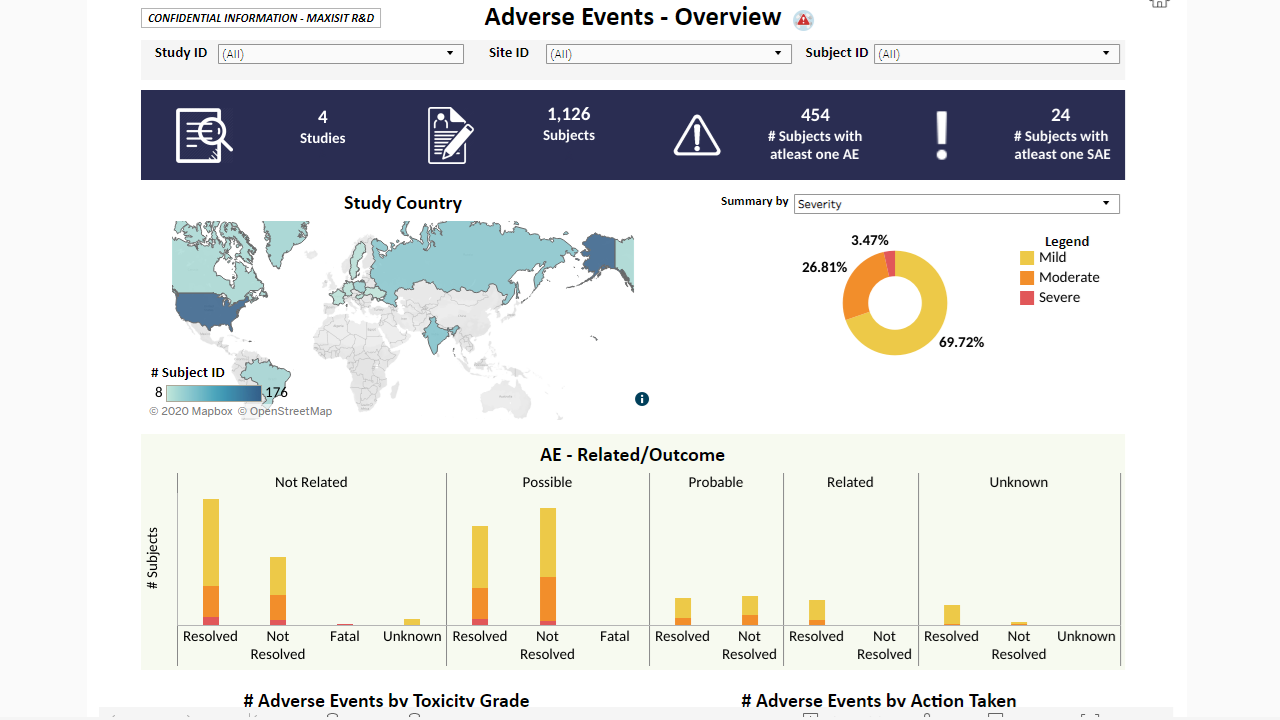
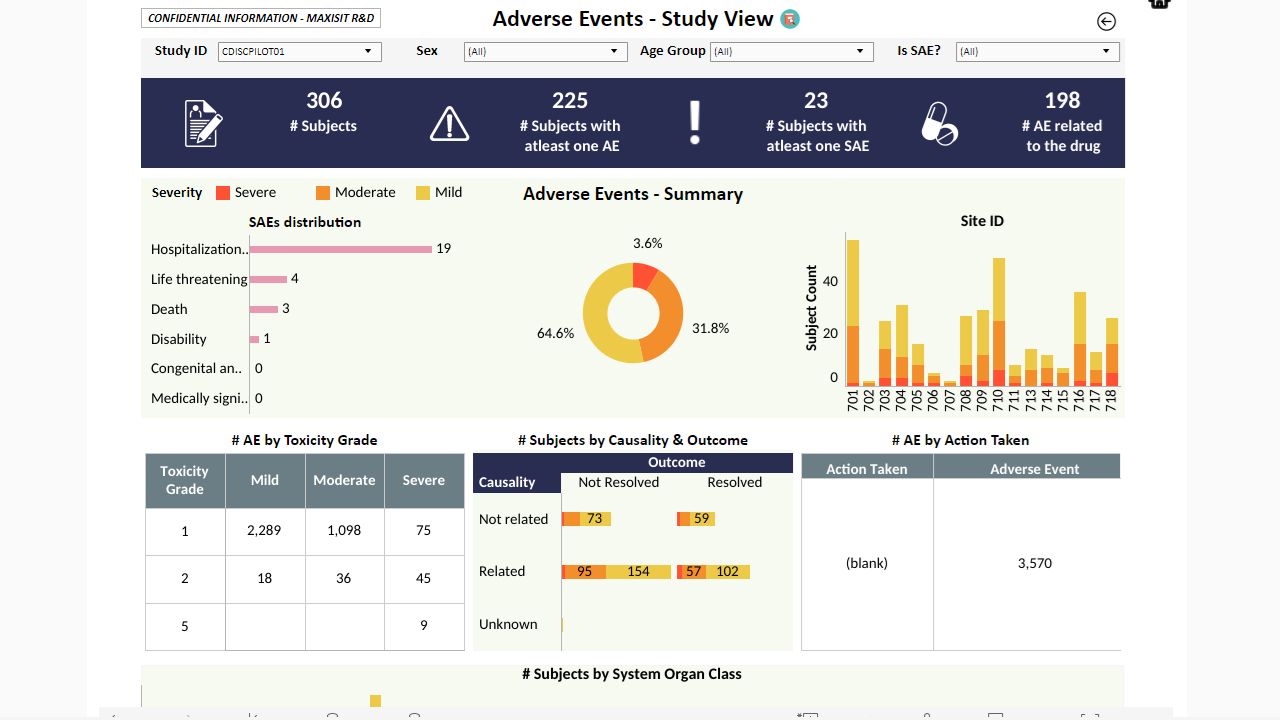
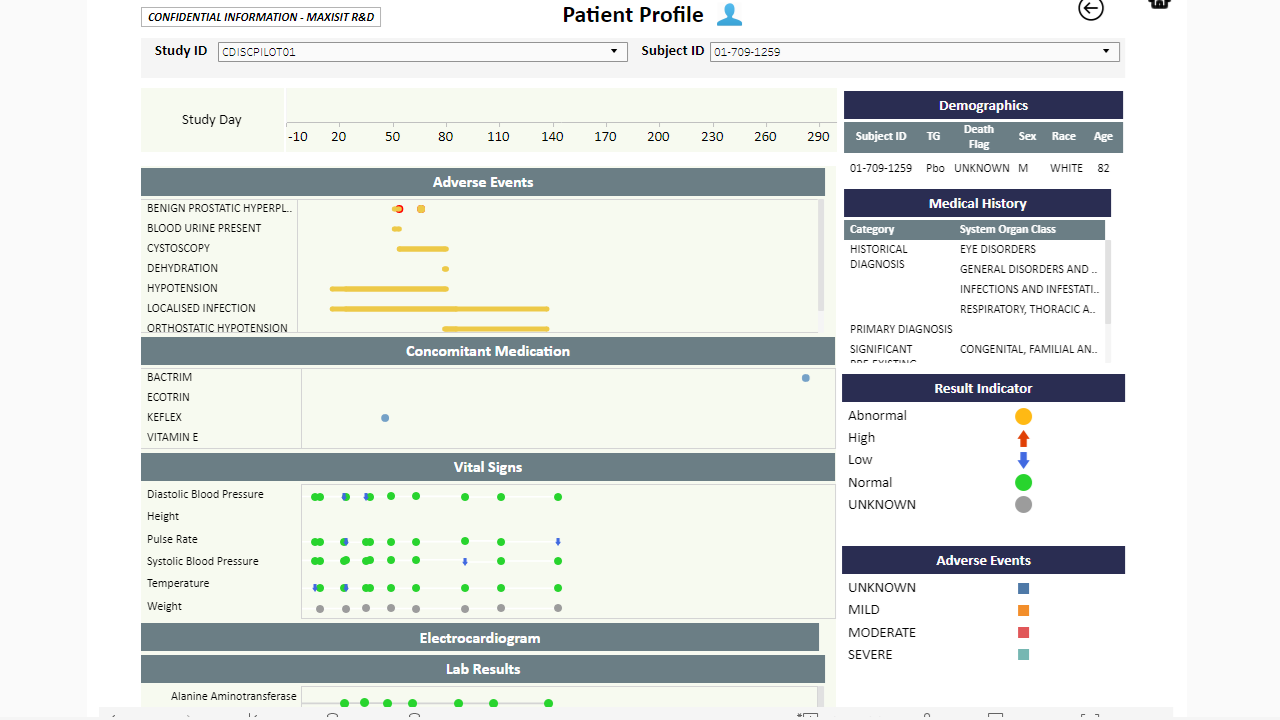
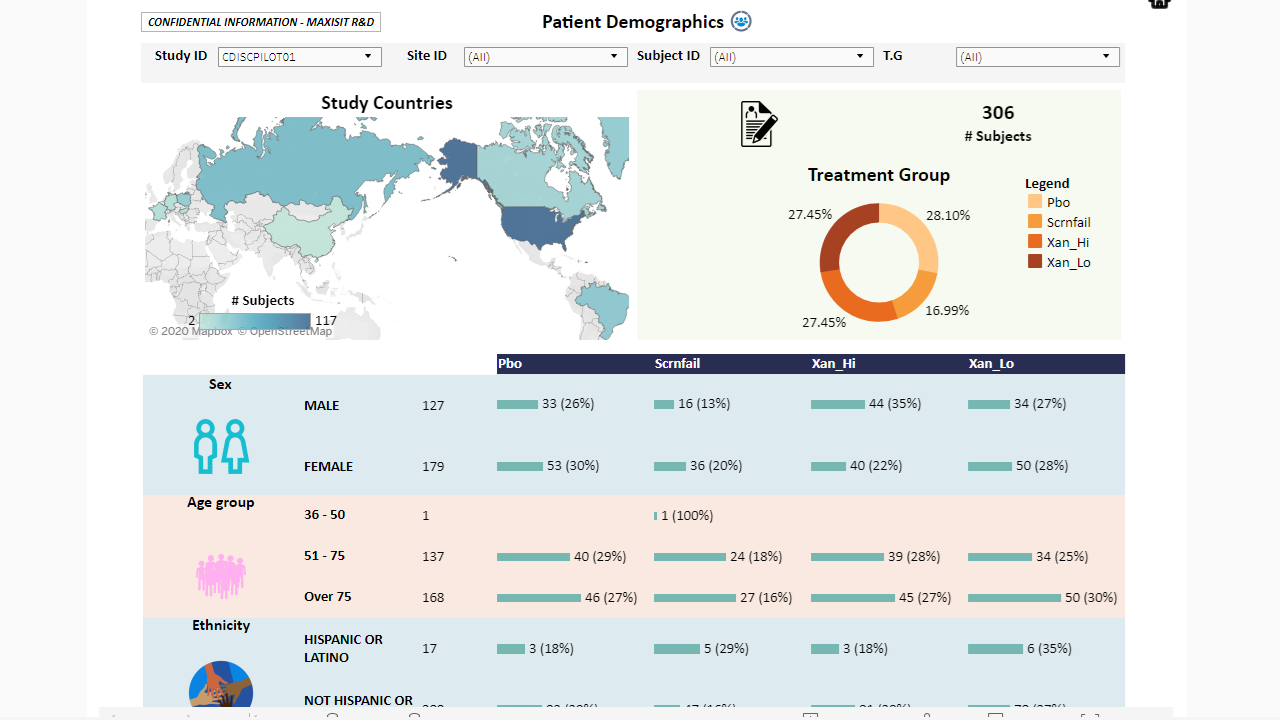
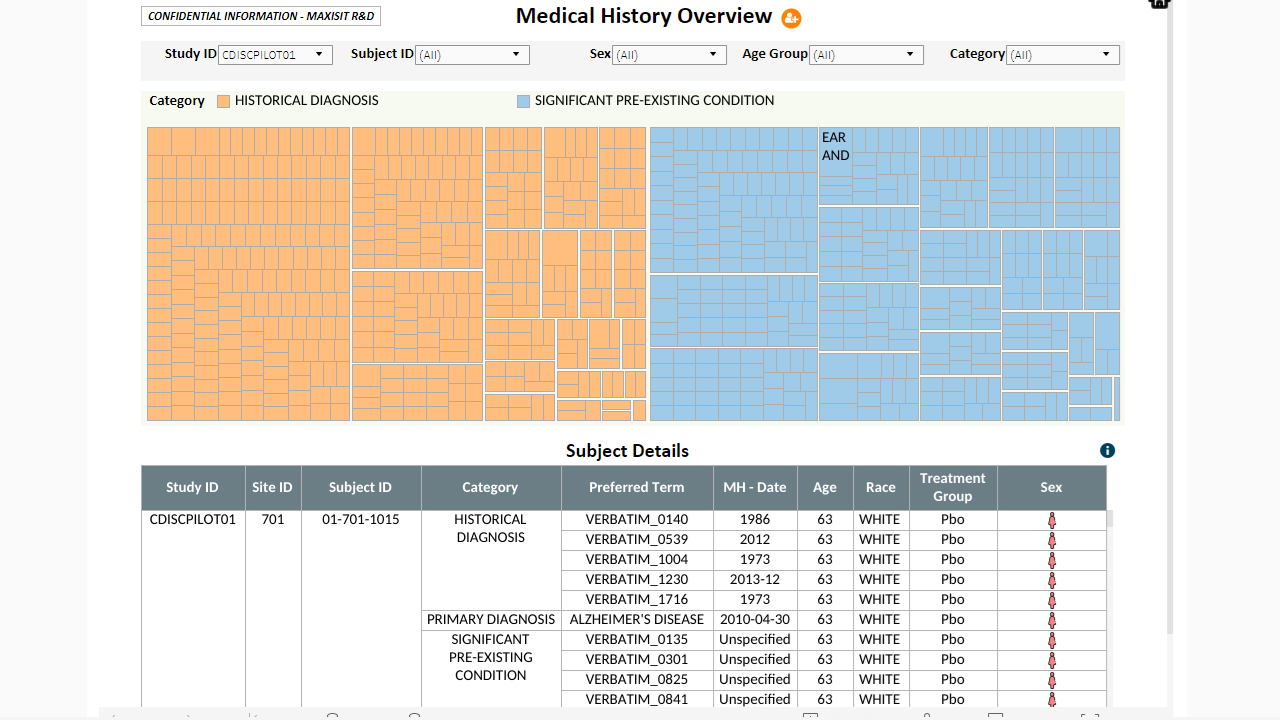
MaxisIT ® is a premier provider of a true cloud-based integrated Platform solution and functional outsourcing services, which are focused on the entire end-to-end life cycle of Clinical Trials and healthcare delivery
Find Us
USA
MaxisIT Inc.
510 Thornall Street, Suite 180, Edison, NJ 08837
Email : info@maxisit.com
Phone : +1-877-MAXISIT (629-4748)
+1 732-494-2005, ext. 135